BRANDS WE OFFER
TENS UNITS
-
EMPI X Direct Tens - 2527116
Regular price $559.00 CADRegular priceUnit price per$649.00 CADSale price $559.00 CADSale -
Primera TENS-NMES with HAN Waveform-by Chattanooga-Made in UK
Regular price $159.99 CADRegular priceUnit price per$179.99 CADSale price $159.99 CADSale -
Chattanooga Cefar Tens
Regular price $364.00 CADRegular priceUnit price per$465.00 CADSale price $364.00 CADSale -
Dr.Ho's 4-pad TENS Muscle Therapy System
Regular price $141.75 CADRegular priceUnit price per$169.00 CADSale price $141.75 CADSale
FEATURED COLLECTIONS
SELECTED COLLECTIONS
TOP CATEGORIES
-

Disposable Massage Supply
Disposable Massage Therapy Supply
FITNESS & REHAB
-
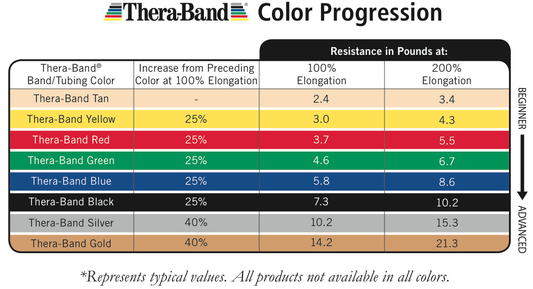
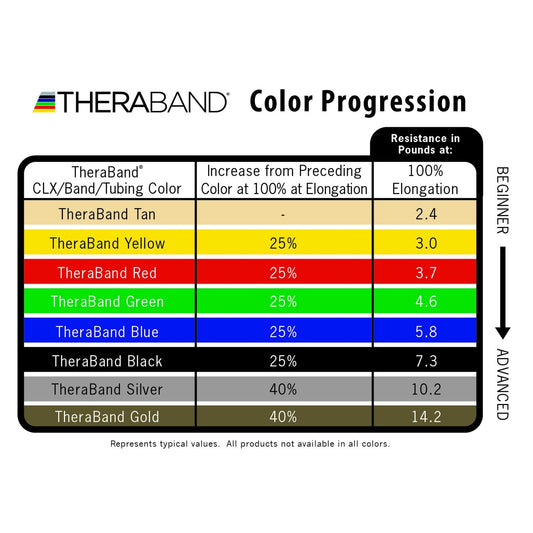
TheraBand Latex-Free Resistance Band 25-Yard Roll
Regular price From $79.00 CADRegular priceUnit price per -
TheraBand Latex-Free Resistance Band 50-Yard Roll
Regular price From $158.00 CADRegular priceUnit price per -
TheraBand Resistance Band Dispenser Packs
Regular price From $169.00 CADRegular priceUnit price per -
TheraBand Latex Resistance Band 50-Yard Roll
Regular price From $129.00 CADRegular priceUnit price per
WHAT'S NEW
-
Hagina Japanese Mint Oil 20ml (4 Pack)
Regular price $78.95 CADRegular priceUnit price per -
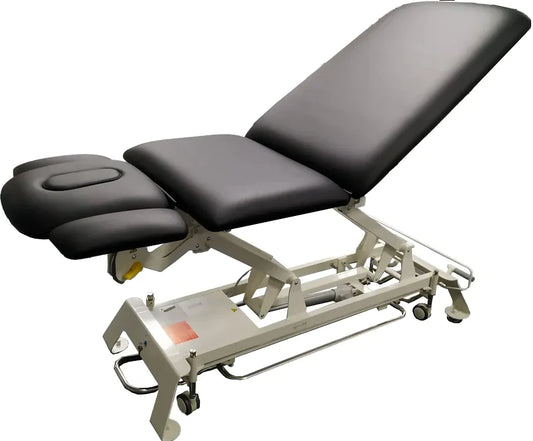
Deluxe 5 Section Treatment Electric Table
Regular price $3,699.00 CADRegular priceUnit price per$3,899.00 CADSale price $3,699.00 CADSale -
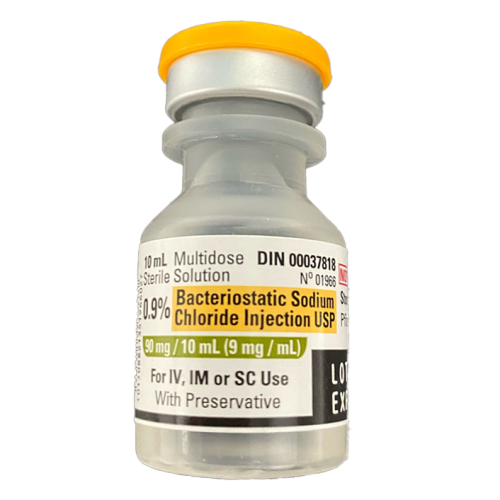
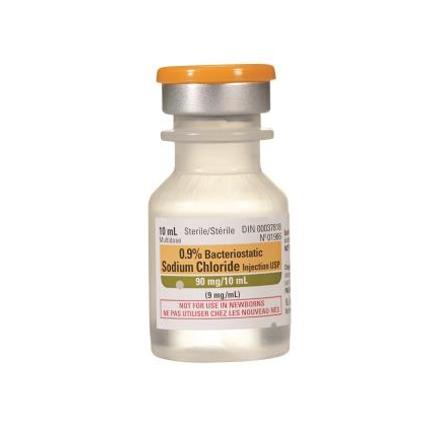
Sodium Chloride 0.9%, 10ml Bottle, Bacteriostatic - With Preservative (2 pack)
Regular price $14.95 CADRegular priceUnit price per$19.95 CADSale price $14.95 CADSale -
Laser Applicators (Fits Vectra Genisys Transport Laser Unit 2784)
Regular price From $1,525.00 CADRegular priceUnit price per